
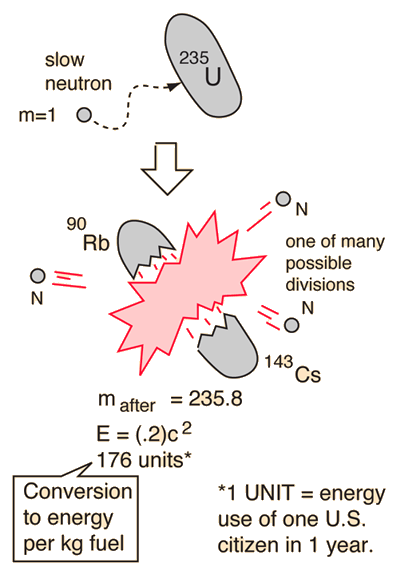
Process (4) also represents the release of a large quantity of energy. For a simple numerical illustration of how this works, click here. Hence there are always a few leftover neutrons, as in Equ. Therefore if a heavy nucleus like uranium splits into two medium-sized nuclei, there are too many neutrons around to fit comfortably on those two nuclei. Why does this happen? Recall that as you go to heavier and heavier nuclei in the nuclear table the number of neutrons relative to protons gets larger and larger. The appearance of extra neutrons is crucial to the matter of nuclear energy, as we will see. Thus a typical fission process might be the following sequence: (The reason we say "on the average" is that fission is occurring in a large number of uranium nuclei, and we have different numbers of neutrons released in different cases, but the average number is 3.) The result was that on the average about 3 neutrons were released per fission.
#Nuclear fission uranium 235 equation free#
Or it may be that in addition to the two medium-sized nuclei produced, there are also some free neutrons released in the fission process.Īround 1940 an important experiment was done (at Columbia University) to determine if free neutrons were emitted in fission, and how many. Where are they? It may be that we really have different Ba and Kr isotopes than the ones given above. If you add up the number of neutrons you find there are 14 missing on the right side. Suppose we hypothesize that the Ba and Kr isotopes in process (1) are the most common naturally found isotopes. The resulting 236U is very unstable, and instead of decaying via some already known form of radioactivity, it decays via fission.

Since fission only occurs when neutrons are present, what really happens is that 235U first absorbs a neutron. For reasons to be discussed below, we will consider the example of fission of 235U. Natural uranium contains the two isotopes, 235U and 238U. This process was called fission, in analogy with the method of reproduction of one-celled animals. And krypton was not there: since it's a gas, it would have just drifted up into the air - not stayed in the uranium sample. Barium was identified via its chemical properties. Element number 36 is krypton, a rare gas. an alpha particle has Z = 2, thorium has Z = 90), here the uranium is split into two approximately equal parts: the second piece must have Z = 92 - 56 = 36. This suggested that a uranium nucleus has split into two parts but unlike normal radioactivity, where one part is very much smaller than the other (e.g. But the elements usually found in this way have high atomic numbers, close to that of uranium. Hahn and Strassman were chemists, and in the tradition of Marie Curie would not have been surprised to discover new elements produced by radioactivity (like thorium). In 1939 Otto Hahn and Fritz Strassman, experimenting with neutrons and a pure sample of uranium, found small traces of the element barium in the sample.


 0 kommentar(er)
0 kommentar(er)
